Scientists probe the chemistry of a single battery electrode particle both inside and out
The results show how a particle’s surface and interior influence each other, an important thing to know when developing more robust batteries.
By Glennda Chui
The particles that make up lithium-ion battery electrodes are microscopic but mighty: They determine how much charge the battery can store, how fast it charges and discharges and how it holds up over time – all crucial for high performance in an electric vehicle or electronic device.
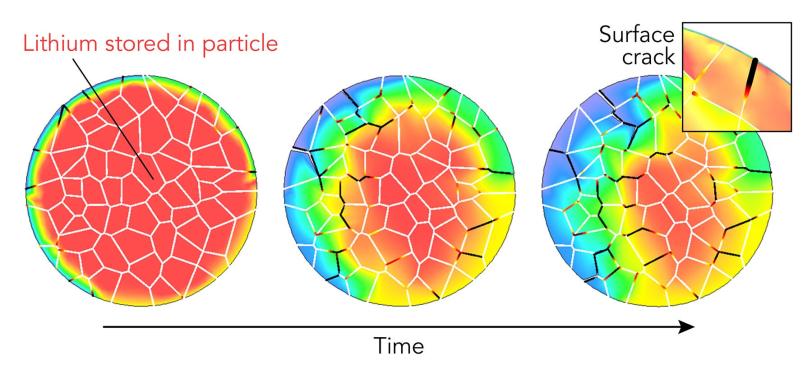
Cracks and chemical reactions on a particle’s surface can degrade performance, and the whole particle’s ability to absorb and release lithium ions also changes over time. Scientists have studied both, but until now they had never looked at both the surface and the interior of an individual particle to see how what happens in one affects the other.
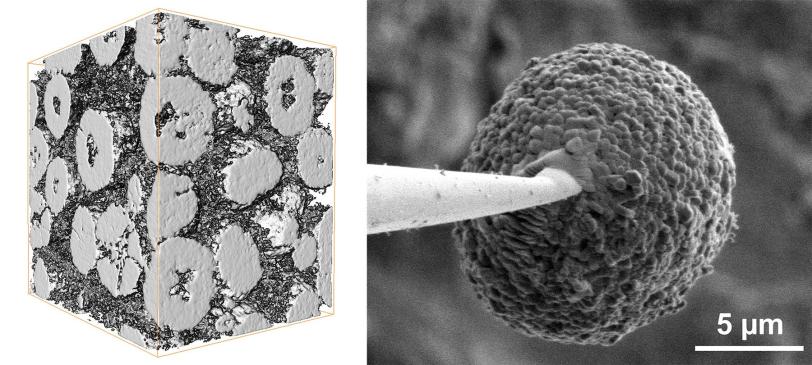
In a new study, a research team led by Yijin Liu at the Department of Energy’s SLAC National Accelerator Laboratory did that. They stuck a single battery cathode particle, about the size of a red blood cell, on a needle tip and probed its surface and interior in 3D with two X-ray instruments. They discovered that cracking and chemical changes on the particle’s surface varied a lot from place to place and corresponded with areas of microscopic cracking deep inside the particle that sapped its capacity for storing energy.
“Our results show that the surface and the interior of a particle talk to each other, basically,” said SLAC lead scientist Yijin Liu, who led the study at the lab’s Stanford Synchrotron Radiation Lightsource (SSRL). ”Understanding this chemical conversation will help us engineer the whole particle so the battery can cycle faster, for instance.”
The scientists describe their findings in Nature Communications today.
Damage both inside and out
A lithium-ion battery stores and releases energy by moving lithium ions through an electrolyte back and forth between two electrodes, the anode and the cathode. When you charge the battery, lithium ions rush into the anode for storage. When you use the battery, the ions leave the anode and flow into the cathode, where they generate a flow of electrical current.
Each electrode consists of many microscopic particles, and each particle contains even smaller grains. Their structure and chemistry are key to the battery’s performance. As the battery charges and discharges, lithium ions seep in and out of the spaces between the particles’ atoms, causing them to swell and shrink. Over time this can crack and break particles, reducing their ability to absorb and release ions. Particles also react with the surrounding electrolyte to form a surface layer that gets in the way of ions entering and leaving. As cracks develop, the electrolyte penetrates deeper to damage the interior.
This study focused on particles made from a nickel-rich layered oxide, which can theoretically store more charge than today’s battery materials. It also contains less cobalt, making it cheaper and less ethically problematic, since some cobalt mining involves inhumane conditions, Liu said.
There’s just one problem: The particles’ capacity for storing charge quickly fades during multiple rounds of high-voltage charging – the type used to fast-charge electric vehicles.
“You have millions of particles in an electrode. Each one is like a rice ball with many grains,” Liu said. “They’re the building blocks of the battery, and each one is unique, just like every person has different characteristics.”
Taming a next-gen material
Liu said scientists have been working on two basic approaches for minimizing damage and increasing the performance of particles: Putting a protective coating on the surface and packing the grains together in different ways to change the internal structure. “Either approach could be effective,” Liu said, “but combining them would be even more effective, and that’s why we have to address the bigger picture.”
Shaofeng Li, a visiting graduate student at SSRL who will be joining SLAC as a postdoctoral researcher, led X-ray experiments that examined a single needle-mounted cathode particle from a charged battery with two instruments – one scanning the surface, the other probing the interior. Based on the results, theorists led by Kejie Zhao, an associate professor at Purdue University, developed a computer model showing how charging would have damaged the particle over a period of 12 minutes and how that damage pattern reflects interactions between the surface and interior.
“The picture we are getting is that there are variations everywhere in the particle,” Liu said. “For instance, certain areas on the surface degrade more than others, and this affects how the interior responds, which in turn makes the surface degrade in a different manner.”
Now, he said, the team plans to apply this technique to other electrode materials they have studied in the past, with particular attention to how charging speed affects damage patterns. “You want to be able to charge your electric car in 10 minutes rather than several hours,” he said, “so this is an important direction for follow-up studies.”
SSRL is a DOE Office of Science user facility. Researchers from Stanford University, Virginia Tech, the Advanced Light Source at DOE’s Lawrence Berkeley National Laboratory and the University of California, Santa Cruz also contributed to the study, which was funded by the DOE Office of Science.
Citation: Shaofeng Li et al., Nature Communications, 7 September 2020 (10.1038/s41467-020-18278-y)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.






